Spontaneity of the acid–base reaction between acetic acid and ammonia... | Download Scientific Diagram

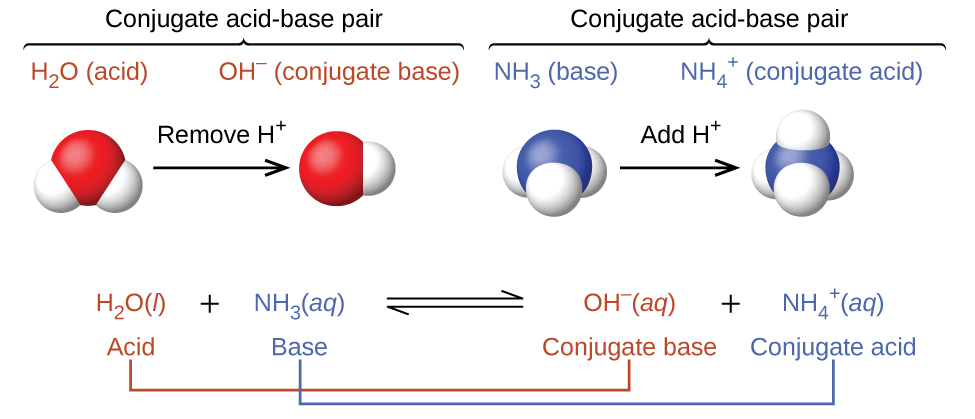
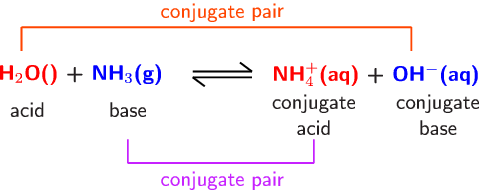
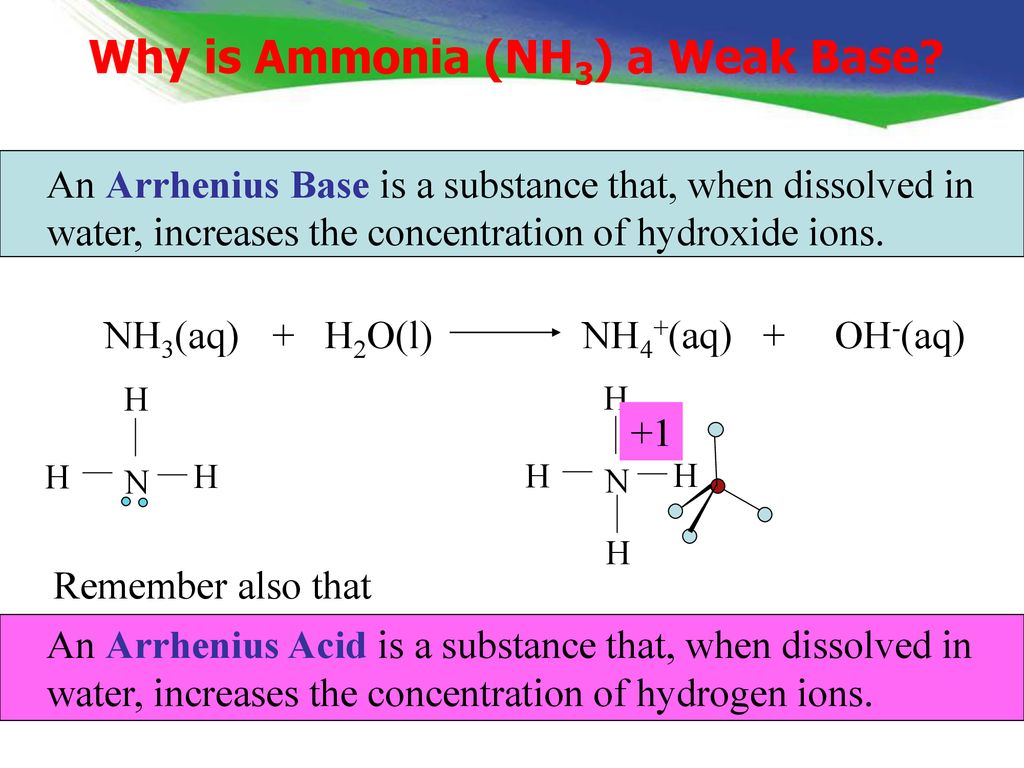
a) Explain why ammonia acts as a weak base in water. (b) Write a balanced chemical equation for the reaction between ammonia and water. | Homework.Study.com

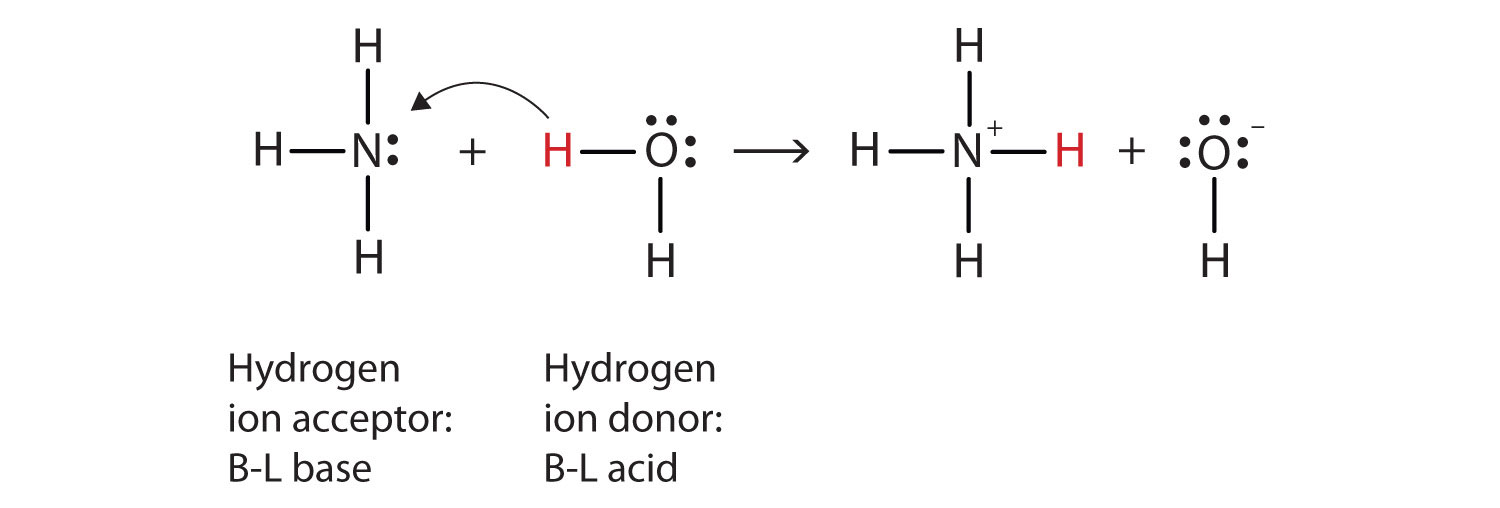
Question Video: Identifying the Lewis Acid in the Reaction of Ammonia with Boron Trifluoride | Nagwa

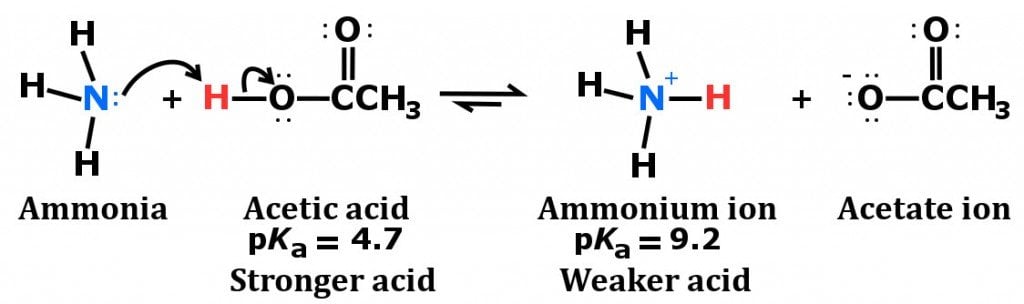
organic chemistry - Why In This Reaction Acetic Acid is strong acid and NH3 is strong base ?please explain in details and thanks for answer - Chemistry Stack Exchange